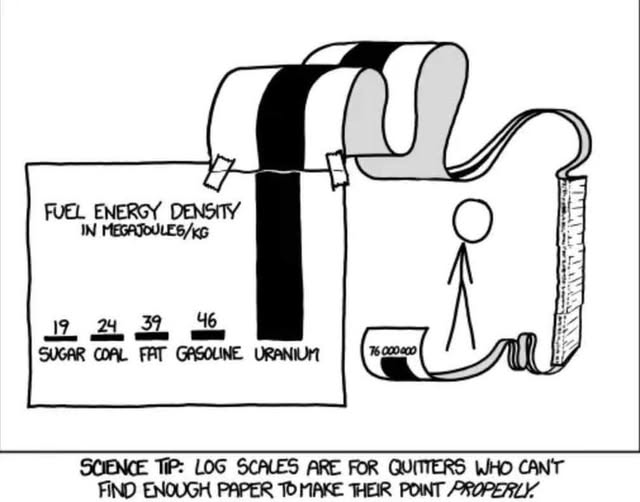
Copy pasta without source. Book! https://xkcd.com/1162/
Science Memes
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- !abiogenesis@mander.xyz
- !animal-behavior@mander.xyz
- !anthropology@mander.xyz
- !arachnology@mander.xyz
- !balconygardening@slrpnk.net
- !biodiversity@mander.xyz
- !biology@mander.xyz
- !biophysics@mander.xyz
- !botany@mander.xyz
- !ecology@mander.xyz
- !entomology@mander.xyz
- !fermentation@mander.xyz
- !herpetology@mander.xyz
- !houseplants@mander.xyz
- !medicine@mander.xyz
- !microscopy@mander.xyz
- !mycology@mander.xyz
- !nudibranchs@mander.xyz
- !nutrition@mander.xyz
- !palaeoecology@mander.xyz
- !palaeontology@mander.xyz
- !photosynthesis@mander.xyz
- !plantid@mander.xyz
- !plants@mander.xyz
- !reptiles and amphibians@mander.xyz
Physical Sciences
- !astronomy@mander.xyz
- !chemistry@mander.xyz
- !earthscience@mander.xyz
- !geography@mander.xyz
- !geospatial@mander.xyz
- !nuclear@mander.xyz
- !physics@mander.xyz
- !quantum-computing@mander.xyz
- !spectroscopy@mander.xyz
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and sports-science@mander.xyz
- !gardening@mander.xyz
- !self sufficiency@mander.xyz
- !soilscience@slrpnk.net
- !terrariums@mander.xyz
- !timelapse@mander.xyz
Memes
Miscellaneous
*Boo
(But having a book instead is always nice.)
I choose to believe it was meant as a warning, because GP is going to yeet a book at your head. But with a fair warning.
I always use “book” as an insult. Especially since my phone autocorrect was updated…
which is bigger? TREE(3) vs
((...(1 room of stacked papers ) room of paper) room of paper)...)) room of paper
The number of brackets in above expression is, eh, ok, you got the idea.
/s
Uranium generates that energy by fission. The hydrogen in sugar could generate huge amounts of energy if fused.
And this boulder could generate huge amounts of energy if I pushed it up to the top of Mt. Kilimanjaro and let it roll down.
44 upvotes and 0 downvotes for a comment that doesn't understand that energy density measurements like this tend to measure the useful energy of a system.
It's disappointing that natural selection didn't figure out fusion.
It figured out photosynthesis instead. Why do your own fusion when you can just take advantage of the fusion that's already happening?
There is still time
I mean, technically it already has.
How much more energy would you get if you fused uranium?
Using the rule of thumb, anything heavier than iron requires energy input to fuse. So you lose energy fusing uranium.
Serious answer: A huge negative amount. Anything above iron requires energy to fuse (which is why it produces energy from fission.) and I'm pretty sure nothing with 184 protons could be stable enough to count as being produced - the nuclei would be more smashed apart than merging at that point.
Whilst I get your point, their point is still valid in the sense that you just can't extract that energy from gasoline in a more efficient manner than just burning it. For practical purposes, gasoline truly is that much less energy dense.
For comparison:
- Chemical combustion of uranium: ~4.7 MJ/kg
- Nuclear fission of uranium-235: ~83.14 TJ/kg (or $ 83.14 \times 10^6 , \text{MJ/kg} $)
and all would generate the same if thrown to something capable of lossless e=mc^2 conversion (maybe a black hole)
sadly black holes go to something like 42% conversion (source: some minute physics video i think)
In theory, yes. In practice, of those two only fission is currently viable.
If we could consume uranium, you could have a teaspoon's worth and be done with eating for the rest of your life.
I think that's technically true regardless.
I wonder if that's actually factual or not. Uranium by itself isn't too terribly dangerous. It's the whole fission byproducts thing that's the buzz kill.
You would get heavy metal poisoning, same as if you ate a chunk of lead

Also it depends on the isotope of uranium. Something you could find naturally isn't too dangerous, but something enriched too be used as fuel or for wepons is significantly more radioactive.
Bah, that graph needs antimatter.
Is there enough paper on earth?
Antimatter doesn't really do anything by it's own, but if we let 1 kg react with 1 kg of matter (non-anti-matter), we get E = mc^2^ with m = 2 kg. So 1.8 * 10^17^ J, or 1.8 * 10^11^ MJ. If we assume that 10 MJ/kg is represented by about 1 cm, the bar would have to be 1.8 * 10^10^ cm or about 1.8 * 10^8^ m. A standard A4 piece of paper is about 30 cm tall, so 6.0 * 10^8^ A4 papers are needed. I.e. 600 million papers.
So we definitely have enough paper, but it would be a very tall stack.
That's only about 180,000km (~112,000 miles) or just under half way to the moon.
Also some quick googling says an average desktop printer can print about 30,000 pages per month, so it would take 20,000 months (~1670 years) to print that out. And a typical toner cartridge can print 3,000 pages and costs $80, so it would take 200,000 toner cartridges and cost $16 million.
Now, those aren't based on any specific model, just the first result in Google haha
Incorrect, if you aren't a bitch about it. Fuse that gasoline!
I was thinking the same thing. It's unfair compare chemical energy to nuclear energy. Coal still kind of sucks, but the hydrogen in the others could definitely be used in fusion...
It is perfectly fair in the context of "fuel", a resource used to produce energy. Whether energy is generated via chemical or nuclear reaction is irrelavent in this case.
Yes boss, I did work out the dynamic range of that log amplifier we wanted to use in our next product's sensor PCB, it's 80dB.
The results are over here. (points to a roll of A-4 paper)
It has 40 data points and only took me 1 week, 10 pencils, and 20 erasers to plot the chart. Yeah I can present it, it'll take me 10 minutes to roll it out, pin it down, and fetch the A-frame ladder.
This is the real big brain hack with decibels
you can use a linear scale, it's just that the units are logarithmic instead.
(Yes I know most people would call a dB axis logarithmic, it's just a silly comment.)
Weird thing I’ve noticed:
Logs are taught in high school. Absolutely no one seems to remember what they are after the unit test, much less high school. I’ve even reminded other math instructors about how to use them.
Why do people have such a hard time learning to use and understand logs?
I love this comic, and it’s going to replace my weird “let’s talk about how this makes the distance between us and Alpha Centauri, and us and Earendil easier to understand” bit.